Io2- lewis structure
The IO2- Lewis structure is a representation of the chemical compound IO2- (Iodine dioxide ion) using Lewis symbols and lines to show the bonding between atoms. Lewis structures are an essential tool in chemistry to visualize the arrangement of atoms and electrons in a molecule or ion. To understand the IO2- Lewis structure, we first need to know the Lewis structure rules and the properties of the IO2- ion. The Lewis structure rules include the octet rule, which states that atoms tend to gain or lose electrons to achieve a stable electron configuration with eight valence electrons. However, some elements, like hydrogen and boron, can have fewer than eight electrons. In the case of the IO2- ion, the central atom is iodine (I) surrounded by two oxygen (O) atoms. Iodine is in Group 7A of the periodic table and has seven valence electrons. Oxygen is in Group 6A and has six valence electrons. Therefore, the total number of valence electrons in IO2- is: 7 (iodine) + 2 × 6 (oxygen) + 1 (negative charge) = 20 To determine the Lewis structure, we start by placing the atoms in a way that satisfies the octet rule. Since iodine is less electronegative than oxygen, it will be the central atom. The oxygen atoms will be bonded to the iodine atom. We can represent this arrangement using a skeletal structure: I O | O Next, we need to distribute the remaining electrons around the atoms to complete their octets. We start by placing two electrons between each iodine-oxygen bond. This leaves 14 electrons to distribute. We place six electrons around each oxygen atom, as they have six valence electrons. Finally, we distribute the remaining two electrons on the iodine atom. The Lewis structure of IO2- is: .. :I-O: :O: In this Lewis structure, the dots represent the valence electrons, and the lines represent covalent bonds. The negative charge is accommodated by the additional electron present in the structure. It is important to note that Lewis structures are not always the most accurate representation of the actual electron distribution in a molecule or ion. They provide a simplified view that helps us understand the bonding and electronic configuration. In reality, the electron density in IO2- is not necessarily localized in double bonds between iodine and oxygen but may be more delocalized. Furthermore, the IO2- ion is not stable in isolation but is typically found as a part of a larger compound or in a chemical reaction. Its stability is due to the interaction with other ions or molecules, which can stabilize the negative charge on the ion. In conclusion, the IO2- Lewis structure is an important tool in chemistry to visualize the arrangement of atoms and electrons in the IO2- ion. It follows the rules of the octet rule and allows us to understand the bonding and electronic configuration of the ion. However, it is important to remember that Lewis structures provide a simplified representation and may not accurately represent the actual electron distribution.
How to Draw the Lewis Dot Structure for IO2 - (Iodite ion) io2- lewis structure. A step-by-step explanation of how to draw the IO2 - Lewis Dot Structure io2- lewis structure. For the IO2 - structure use the periodic table to find the total number of valence electro Energy Levels, Energy.. Lewis Structure of IO2- (With 6 Simple Steps to Draw!) - Knords Learninghot internet dating info adult site
. It is an iodine oxoanion and a monovalent inorganic anion.. IO2- Lewis Structure, Characteristics:11 Facts You Should Know. IO2- is an oxyanion halogen of Iodine and Iodine bears a negative charge in the IO2- lewis structure. It is bent shape due to the presence of lone pair. The lone pair-bond pair repulsion makes the geometry of IO2- bent like a water molecule io2- lewis structure. Let us focus on some important facts about IO2- like, lone pairs, valence electrons, hybridization. 1. io2- lewis structure. 9.3: Drawing Lewis Structures - Chemistry LibreTexts io2- lewis structure. Rule 1: In any molecule or ion with the general formula ABn , the unique atom (A) is in the center and all of the B atoms are attached to A. Rule 2: Lewis structures are not intended to show the actual shape of the molecule; they only show which atoms are bonded to each other.. IO2- Lewis structure - Learnool. In IO 2- Lewis structure, there is one single bond and one double bond around the iodine atom, with two oxygen atoms attached to it io2- lewis structure. The oxygen atom with a single bond has three lone pairs, the oxygen atom with a double bond has two lone pairs, and the iodine atom also has two lone pairs.. iodite ion | IO2 | ChemSpider
free symbicort samples
. Electrons are shown as "dots" or for bonding electrons as a line between the two atomsson forcly fuck mom dont want fuck
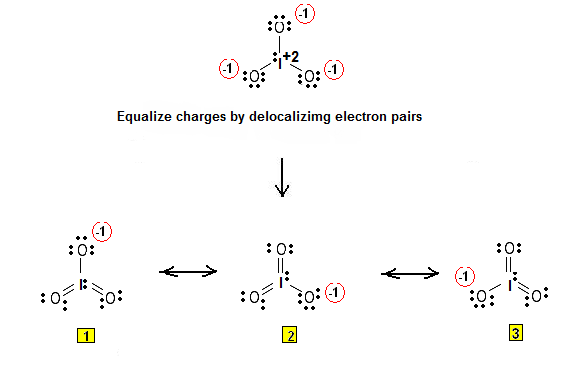
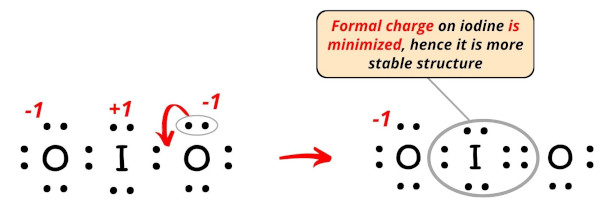
. The goal is to obtain the "best" electron .
tongue clicking noise crossword
. شکل ساختار IO2- lewis خطی نیست. شکل در اطراف اتم مرکزی کمی خم شده است

desmos polar coordinates
. periodate is an inorganic mono - valent negative ion (anion). io2- lewis structureshake n go
. What is the Lewis structure of IO2-? - Answers io2- lewis structure. Q: What is the Lewis structure of IO2-? Write your answer. Still have questions? Find more answers Ask your question Continue Learning about Chemistry What is the chemical compound name for.. Solved Draw a Lewis structure for IO2- (I is in the middle) - Chegg. Draw a Lewis structure for IO2- (I is in the middle) that puts a negative one formal charge on one O, and that has zero formal charge for the other O and that has zero formal charge for I io2- lewis structure. How many lone pairs (unshared pairs) are on central atom? [ O I O ]- 1 2 3 This problem has been solved! io2- lewis structure. IO2- lewis structure, molecular geometry, bond angles, polarity. The Lewis dot structure of the iodite (IO2-) ion comprises an iodine (I) atom at the centerdirty garage fuck! quick. hot. hard.
. It is surrounded by two oxygen (O) atoms, one on either side, via single and double covalent bonds, respectively.. IO3- lewis structure: Drawings, Hybridization, Shape, Charges, Pairs. By Susanta Maity In this article we are discussing about io3- lewis structure including its drawing, hybridization, shape, pairs and some FAQS. Iodate is an oxoanion of iodine. It is formed when Iodic acid losses one proton. It has the molecular weight of 174.903